
J-GMP
GMP Inspection concerning Pharmaceuticals (including APIs) of Foreign Manufacturers
Pharmaceuticals and Medical Devices Agency
History
Following the Reorganization and Rationalization Plan for Special Public Corporations that was approved in a Cabinet meeting in 2001, the Pharmaceuticals and Medical Devices Agency (PMDA) was established and came into service on April 1, 2004, under the Law for the Pharmaceuticals and Medical Devices Agency, as a consolidation of the services of the Pharmaceuticals and Medical Devices Evaluation Center of the National Institute of Health Sciences (PMDEC), the Organization for Pharmaceutical Safety and Research (OPSR/KIKO), and part of the Japan Association for the Advancement of Medical Equipment (JAAME).
Service

Pharmaceuticals and Medical Devices Agency (Guidance for Foreign Manufacturers)
GMP Compliance Inspection concerning Pharmaceuticals of Foreign Manufacturers is an inspection on the compliance of manufacturing control and quality control methods at the relevant manufacturing sites with Japanese GMP (“Ministerial Ordinance on Standards for Manufacturing Control and Quality Control for Drugs and Quasi-drugs”, Ordinance of Ministry of Health, Labor and Welfare, No. 179, 2004), conducted by the Pharmaceuticals and Medical Devices Agency (hereinafter “PMDA”). GMP compliance is a requirement for marketing approval.
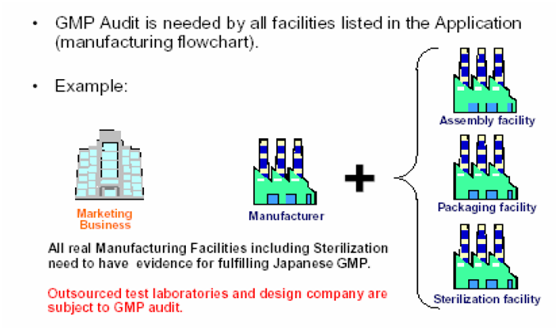
GMP Compliance Inspections include 1) Inspections that are conducted at the point of application for new marketing approval or of application for partial changes of approved information, and 2) Inspections that are conducted every five years following the obtainment of marketing approval. In the case of ethical drugs, packaging, labeling and storage facilities and external testing laboratories are included in the scope of GMP Inspection, in addition to the manufacturing sites of drug products, APIs (Active Pharmaceutical Ingredients) and intermediates. In the case of application for partial change approval, GMP Compliance Inspection is not required if the partial change is addition, change, or deletion etc. of administration and dosage, or indication that will not affect the methods for manufacturing control or quality control. While drug products for over-the-counter drugs are included in the scope of GMP compliance Inspection, APIs for over-the-counter drugs are excluded from the inspection (however APIs of over-the-counter for new marketing approval are in the scope of GMP compliance Inspection).
A marketing authorization holder that applies for the marketing approval of pharmaceutical, or an appointed marketing authorization holder designated by a manufacturer that seeks to obtain foreign restrictive approval, shall file an application with the PMDA for GMP compliance Inspection of foreign manufacturing sites.
Following the application for GMP Compliance Inspection, the applicant shall submit ‘Documents pertaining to manufacturing control and quality control of product(s) concerning the compliance inspection” and “Documents pertaining to manufacturing control and quality control of manufacturing sites concerning the compliance inspection”, at request of the PMDA.
Even applications and attached documents are concerning foreign manufacturing sites, it should be prepared in the Japanese language. If the attachment includes a large volume of documents written in a foreign language, it is acceptable to prepare only an overview of such documents in Japanese.
Who will be J-GMP inspected?
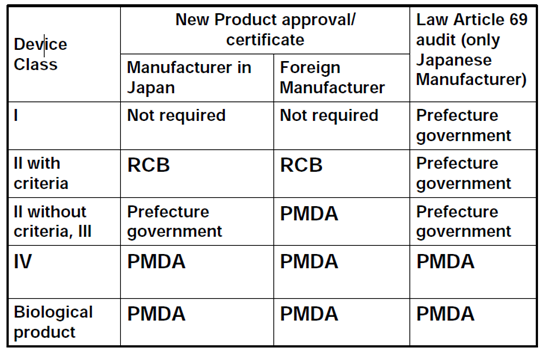
Who executes J-GMP inspection?
GMP / QMS / GCTP Inspections
When drug products, medical devices or cellular and tissue-based products are manufactured, all product batches should be of the same quality as that of the product which is approved. To ensure this, the manufacturing site should have appropriate manufacturing facilities, and the manufacturing process and quality management system should be maintained and controlled properly.
GMP inspection (products classified as “high-risk”)
For GMP inspection, PMDA conducts on-site and document- based inspections of manufacturing sites for products classified as “high-risk,” such as new drugs, biological products or biotechnological products (including foreign manufacturing sites), in order to ascertain whether their manufacturing facilities and manufacturing and quality controls comply with standards such as the Good Manufacturing Practice (GMP), and whether the manufacturing sites have a system for manufacturing products of adequate quality.
PMDA also conducts inspections in relation to accreditation of foreign manufacturers.
QMS inspection (products under review or approved products)
For medical devices and in vitro diagnostics, PMDA conducts on-site and document-based inspections of the registered manufacturing sites (of products under review or approved products) located in Japan or overseas, in order to ascertain whether their manufacturing facilities and manufacturing and quality controls comply with standards such as the Quality Management System (QMS), and whether the manufacturing sites have a system for manufacturing products of adequate quality.
GCTP inspection (cellular and tissue-based products)
PMDA has established a system to inspect manufacturing sites of cellular and tissue-based products located in Japan or overseas, in order to determine whether their manufacturing facilities as well as manufacturing process and quality management system comply with the Good Gene, Cellular, and Tissue-based Products Manufacturing Practice (GCTP).
PMDA has also developed a necessary system for inspections on compliance with the standards for buildings and facilities, and for-cause inspections and questioning for cell processing facilities, which will be newly started by the enforcement of the Act on Securing Safety of Regenerative Medicine.
What We Do?
--Gap analysis audit
--Pre-assessment audit
--Full or partial internal audit
--Subcontractor or supplier audit
--Document and records control
--Training on regulatory requirements and internal procedures
--Design and development
--Risk management
--Software development (if applicable)
--Supplier evaluation
--Control of measurement equipment
Why Jiushun Management?
Jiushun Management, focused on Medical Device Registration, Certification for 20 years, provided high quality service for more than 5,000 global customers.
The MD industry focus degrees, regulatory familiarity, and rich experience, determine our high efficiency and professionalism.
Our professional technical support, from the early regulations, processes guidance, documentation preparation, to testing assistance, review tracking, as well as years of MD industry resource integration, will greatly shorten the period of your product approval, and well practice our principle "Jiushun Management, create value for you. ".





